Malaria
THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH (WEHI)
Melbourne, Australia
Identifying and disrupting key elements of malaria's "sticky sack" adhesion strategy
Malaria is one of the most devastating diseases afflicting humanity. It infects and debilitates about 600 million people and kills up to three million people every year, mainly in the wet tropical regions of the world. Children and pregnant women are at particularly high risk.
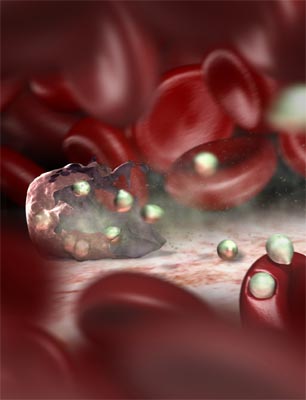
The malaria parasite is injected into humans by an infected mosquito. The parasites then infect healthy red blood cells, transforming them into sticky sacks containing up to thirty-two new daughter parasites. The hijacked red blood cells stick to blood vessel walls, thereby avoiding being flushed through the spleen and being destroyed there by the body's immune system.
WEHI scientists have revealed key elements in the parasite's "sticky sack" adhesion strategy. They have identified eight new proteins that transport the parasite's major adhesion factor, PfEMP1, to the surface of infected red cells, where it promotes the formation of sticky knobs. They have also shown that removal of just one of these proteins disrupts the ability of the parasite bag to stick to blood vessel walls.
This discovery has greatly enhanced our understanding of how the parasite commandeers the red blood cell for its own survival and avoids our immune defences. It also suggests that a drug that inactivates an essential adhesion protein would be an effective anti-malarial.
All currently available malaria drugs attempt to disrupt the metabolism or biological function of the parasite. Unfortunately, malaria parasites are evolving resistance to such drugs, suggesting that quite a different strategy may be required - hence the importance of targeting the "stickiness factors." The inability of the parasite to prevent its transport to the human spleen would lead to the parasite's natural destruction.
The team of WEHI-based and international collaborators includes Alan Cowman, Alex Maier, Melanie Rug, Matthew O'Neill, Monica Brown, Srabasti Chakravorty, Tadge Szestak, Joanne Chesson, Yang Wu, Katie Hughes, Ross Coppel, Chris Newbold, James Beeson, Alister Craig and Brendan Crabb.
The work was supported by the Wellcome Trust, the NHMRC, the Howard Hughes Medical Institute and the National Institutes of Health.
The findings were published in the prestigious international journal, Cell.
Melbourne, Australia
Identifying and disrupting key elements of malaria's "sticky sack" adhesion strategy
Malaria is one of the most devastating diseases afflicting humanity. It infects and debilitates about 600 million people and kills up to three million people every year, mainly in the wet tropical regions of the world. Children and pregnant women are at particularly high risk.
The malaria parasite is injected into humans by an infected mosquito. The parasites then infect healthy red blood cells, transforming them into sticky sacks containing up to thirty-two new daughter parasites. The hijacked red blood cells stick to blood vessel walls, thereby avoiding being flushed through the spleen and being destroyed there by the body's immune system.
WEHI scientists have revealed key elements in the parasite's "sticky sack" adhesion strategy. They have identified eight new proteins that transport the parasite's major adhesion factor, PfEMP1, to the surface of infected red cells, where it promotes the formation of sticky knobs. They have also shown that removal of just one of these proteins disrupts the ability of the parasite bag to stick to blood vessel walls.
This discovery has greatly enhanced our understanding of how the parasite commandeers the red blood cell for its own survival and avoids our immune defences. It also suggests that a drug that inactivates an essential adhesion protein would be an effective anti-malarial.
All currently available malaria drugs attempt to disrupt the metabolism or biological function of the parasite. Unfortunately, malaria parasites are evolving resistance to such drugs, suggesting that quite a different strategy may be required - hence the importance of targeting the "stickiness factors." The inability of the parasite to prevent its transport to the human spleen would lead to the parasite's natural destruction.
The team of WEHI-based and international collaborators includes Alan Cowman, Alex Maier, Melanie Rug, Matthew O'Neill, Monica Brown, Srabasti Chakravorty, Tadge Szestak, Joanne Chesson, Yang Wu, Katie Hughes, Ross Coppel, Chris Newbold, James Beeson, Alister Craig and Brendan Crabb.
The work was supported by the Wellcome Trust, the NHMRC, the Howard Hughes Medical Institute and the National Institutes of Health.
The findings were published in the prestigious international journal, Cell.
MORE
- Melanoma treatment
- Positive
- Chickenpox Vaccination
- Treatment for Advanced Liver Cancer
- Genetic link to depression in Huntington's disease
- Electronic Prescriptions
- Aussies Waking Up to Allergies
- managing type 2 diabetes
- Kidney Watch Early Detection
- Kidney Watch High Blood Pressure
- Sleep Loss and Daytime Performance



